Dimethyl Sulfide to Sulfuric Acid: A DFT Study of the Oxidation and Isomerization of DMS
Author
Bruce Prince — Texas Southern University *
Category
Secondary organic aerosol
Description
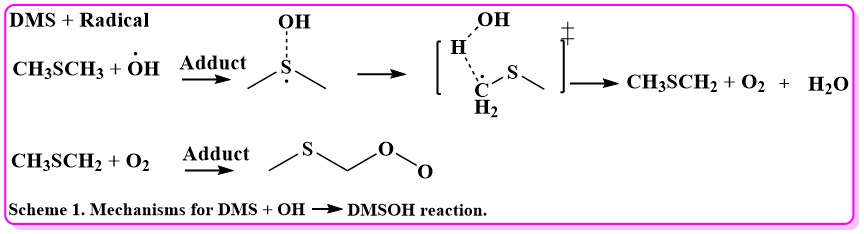
Volatile organic compounds (VOCs) (e.g., dimethyl sulfide (DMS), hydroxyl radical, methoxy radical, methane gas, carbon monoxide, carbon dioxide, sulfuric acid, water gases, etc.) serve as cloud condensation nuclei and have a substantial effect on cloud properties and the initiation of precipitation (rain) and elevated temperature. Hence, volatile organic compounds (VOCs) play a key role in Earth's precipitation and solar energy-radiation cycle. However, despite extensive studies, the chemical and physical processes that govern these VOC (Volatile organic compounds) interactions remain scarce and sometimes ambiguous representing a major source of uncertainty in predictive cloud modeling theories. Our current research will focus specifically on the emission of dimethyl sulfide (DMS: CH3SCH3). DMS is one of the largest natural sulfur sources to our environment with an estimated rate of 2.3 x 106 metric tons of sulfur/year. The principal focus of this preliminary investigation is trying to answer the particularly important question, what is the primary pathway to sulfuric acid (H2SO4) from DMS. The cycle of DMS to H2SO4 processes play significant roles also in climate regulation because of the formation of sulfate in marine aerosols and cloud condensation nuclei (CCN).
* This research is supported, in part, by DOE RDPP grant DE-FOA-0002688.