With a 2019 pilot study on the books and a rich trove of data, scientists close in on how to represent ice in models
If you find yourself driving along a quiet roadway in Long Island, New York, you may see a figure in full Tour de France regalia whizzing by on a road bike. That would be atmospheric scientist Daniel Knopf, a professor at Stony Brook University in New York. Outside of work, when time allows, he bikes, hikes, and skis―interests he acquired growing up near Mannheim in southwestern Germany.
A couple of decades ago, Knopf turned the lens of his classical European education in physics and chemistry to focus on aerosol particles. These ultrafine bits, as small as 2 nanometers in diameter, are suspensions of both liquid and solid particles in a gas. Their physical and chemical properties―and the phase changes they undergo in the atmosphere―influence cloud formation, precipitation, and the surface energy budget of the Earth.
Knopf and his research group pursue a wide range of aerosol-related interests, including imaging analysis, particle atmospheric transport and chemical transformation, aerosols from sea spray and blue-green algae, and instrument development. (In collaboration with Pavlos Kollias of Stony Brook and Brookhaven National Laboratory, they recently crafted an octocopter drone capable of an 8-kilogram―17.6-pound― instrument payload for collecting particulates and analyzing trace gases.)
The Knopf group has a particularly intense interest in how ice forms in clouds. Without ice in clouds, little or no precipitation would fall in Earth’s middle latitudes.
For several years they have been working on the best way to represent this important process in models. It is challenging to map out the ice-formation process, however. Aerosol particles, moving air, and cloud microphysics interact in very complex ways.
In all, writes Knopf in an October 2021 paper, predicting how ice forms in clouds “is one of the grand challenges in the atmospheric sciences.”
Ice Nucleating Particles
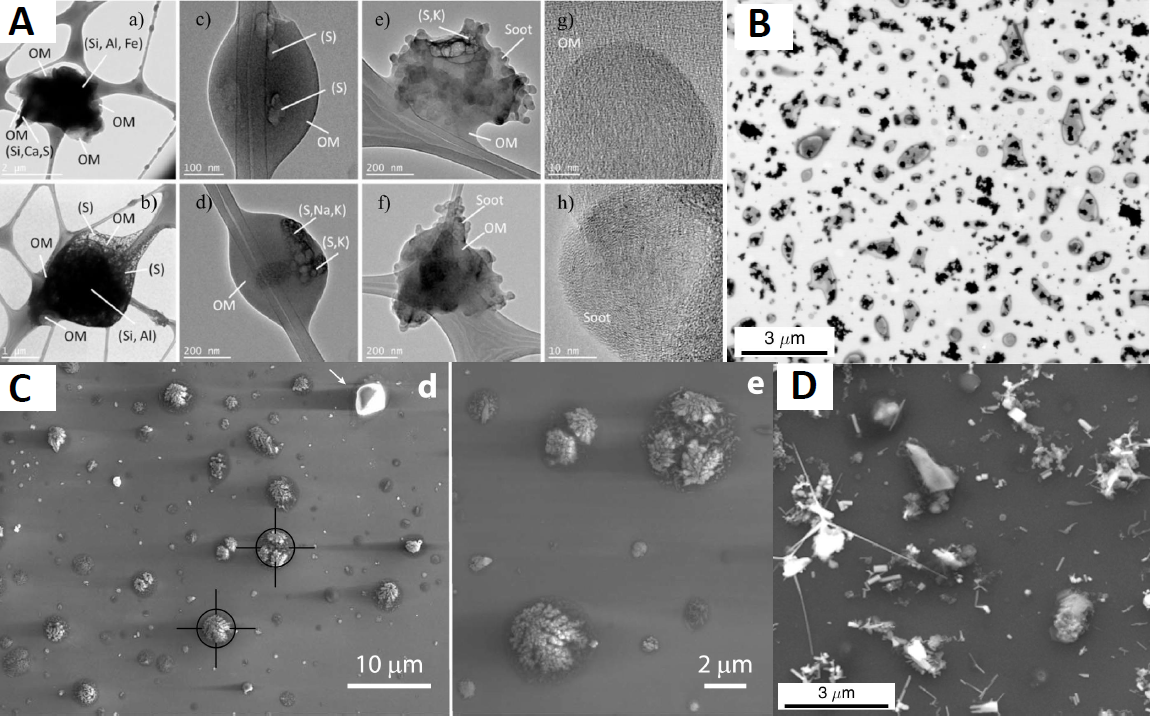
Within the grand challenge of ice formation is the puzzle of ice-nucleating particles (INP). These elusive, seed-like bits of inorganic and organic matter are the key to forming ice crystals.
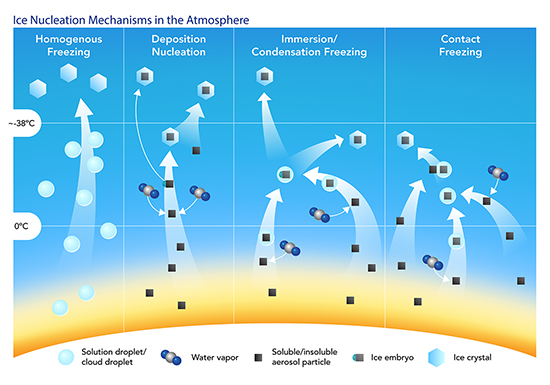
Knopf leads a 2021―2023 research project designed to improve the predictive understanding of ice-crystal number concentration in mixed-phase clouds. Those are the kind in which supercooled water droplets and ice crystals exist side-by-side, a condition of fruitful instability that makes precipitation possible.
The instability arises because an ice crystal grows by diffusing―stealing―water vapor from the drops. Once the ice crystal is larger than the drops, it falls, colliding with more water droplets along the way―collisions that make snow, graupel, hail, and rain possible. Such precipitation, the elixir of life and fundament of the water cycle, emerges from the base of clouds.
However, the vital chain of actions that leads from aerosols to INP and then to concentrations of ice crystals and to precipitation is hard for observationists to measure and for modelers to represent.
“There are many more waterdrops than ice crystals,” says Knopf, who studies how ice crystals form via heterogeneous nucleation―the kind that initiates only in the presence of a foreign substance. “Most rain is a consequence of ice crystals, yet only a few aerosol particles can initiate ice crystal formation in clouds.”
His research project is funded by the Atmospheric Research System (ASR) program at the U.S. Department of Energy (DOE).
Knopf was also the science lead for a related field campaign enabled by DOE’s Atmospheric Radiation Measurement (ARM) user facility, which collects continuous data in the world’s climate-critical regions. His multi-agency, multi-university Aerosol-Ice Formation Closure Pilot Study (supported by both ASR and ARM) took place over three weeks in the fall of 2019 at ARM’s Southern Great Plains (SGP) atmospheric observatory, which spans parts of rural Oklahoma and Kansas.
“There are many more waterdrops than ice crystals. Most rain is a consequence of ice crystals, yet only a few aerosol particles can initiate ice crystal formation in clouds.” – Daniel Knopf
The October 2021 paper lays out some preliminary results and a campaign overview.
The aim of the fieldwork at SGP was to create closure―that is, to close the gap between INP observations and the predicted rates of ice formation by aerosols in ambient air. Which particles do the work of ice formation, and at what rates? Finding out would be a boon to modelers, who need the improved parameterizations (simplified representations) of ice formation that Knopf and others strive to provide.
“It’s important,” he says, “if you want to improve our prediction of clouds and precipitation.”
‘Better and More Completely’
What’s next with the closure campaign “could be interesting for ASR,” says Knopf, since preliminary results from the pilot study point to the need for a larger exercise. “Now we know how to do it better and more completely.”
Meanwhile, he is full of gratitude for the pilot campaign in 2019 that churned up novel data and that―in a unifying way, says Knopf―brought together a range of scientists, each “with different ideas about ice nucleation. Without ASR and ARM, we could not work in that way together. Now we can break (disciplinary) boundaries.”
And meanwhile, too, he says, “aerosol, aerosol-ice cloud, and ice crystal folks” will get together to imagine the outlines of a bigger campaign and discuss what expertise and instruments were missing the first time around.
For now, “we achieved a lot for a pilot study,” says Knopf. “Everyone was flabbergasted.”
Those achievements were outlined in an October 2020 campaign report and discussed in more detail in the October 2021 study, which appears in the Bulletin of the American Meteorological Society.
Closure on the issue of immersion freezing from ambient aerosol particles is close, the Knopf-led paper says, calling the pilot study “a robust foundation for guiding the representation of INPs in cloud and climate models.” But roadblocks remain: a physiochemically complex aerosol population, for one, and a lack of parameterization for some INP types―especially, perhaps, because of what he calls “insufficient knowledge of biological particles.”
Still, says Knopf in the aftermath of his pilot study, “the overall trajectory is clear. We know how to go about it now.”
Smashing Heavy Ions

“I love the mountains, I love the snow,” says Knopf of his outdoor experiences, from childhood on, that underlay an early interest in science. “I was always fascinated by the phases and aggregates of snow” and the complexities behind its creation in clouds.
He describes himself as a “classically trained physicist,” whose first training was at Ruprecht-Karls University (Heidelberg University) in Germany (B.Sc., 1994; supplemental studies in environmental science, 1996; M. Sci, 1999).
At Heidelberg, famous for its high-energy physics, Knopf was drawn to both the world of atmospheric physics and that of particle physics.
For the latter, he attended classes and seminars by experimental physicist Johanna Stachel, a former president of the German Physical Society, whose work focused on heavy-ion collisions.
At high enough collision energies in a particle accelerator, smashing one heavy ion against another at close to the speed of light can produce quark-gluon plasma―the substance thought to comprise the entire universe before matter was created.
“She was inspirational,” says Knopf of Stachel, and the subject matter was heady stuff: explorations of the fundamental forces of the universe and of elementary particles (subatomic particles comprised of no other particles).
“At the time, I was very far from Earth” in a way, says Knopf of his intense foray into particle physics. “I was captivated―but then I came back to the snow and clouds,” that is, to atmospheric physics.
Knopf was also interested in environmental physics and he credits Ulrich Platt, an environmental physicist at Heidelberg, for his classes on stratospheric ozone depletion and polar stratospheric clouds.
He looks at atmospheric physics today as “something like high energy physics,” with principles governing complex interactions, correlations, shapes, and movements “that are up there―still unreachable, though visible and less abstract as high energy physics.”
Back to Visible Nature
When Knopf did his master’s degree thesis at Heidelberg, he needed to choose between a high-energy physics and atmospheric sciences topic. Choosing between the two directions, he reflects, “was a really hard choice. It was almost emotional.”
His master’s degree work on polar stratospheric clouds took place at the Max Planck Institute for Nuclear Physics, which is also in Heidelberg, Germany. It was there that Knopf met an important intellectual influence: Konrad Mauersberger, an atmospheric physicist who pioneered an aerial mass-spectrometry platform borne into the sky on balloons, which enabled the analysis of polar stratospheric clouds in the stratosphere. He had returned to his native Germany to direct the Institute after a 25-year stay at the University of Minnesota.
“He was so humble, wise, and charismatic,” says Knopf, who when offered a hard-to-get spot on the Mauersberger research team “could not say ‘No.’ I was smitten.”
But polar stratospheric clouds, in the end, were the means of coming back to visible nature.
From there, says Knopf, “I worked my way down in the atmosphere” until, in the present day, he studies the aerosols that whirl low in the atmosphere’s boundary layer.
Even though Knopf entered Heidelberg University as a teen-ager, he was aware of the science banquet laid out for him. He tried a main course of both chemistry and chemical engineering before finally settling on physics and studies of the atmosphere.
In particular, “I realized physics would give me a lot. It would allow me to look at the world.”
Switzerland, British Columbia and Beyond

Knopf intensified that look at the natural world in Zurich, Switzerland, with doctoral studies at the Institute for Atmospheric and Climate Science at the Swiss Federal Institute of Technology (PhD, 2003).
He was the first PhD student of atmospheric chemist Thomas Koop (now at Bielefeld University) and was co-advised by Thomas Peter, an expert in tropospheric chemistry. Knopf learned more about aerosol phase transitions and, in particular, was introduced to atmospheric ice formation.
“Thomas Koop was such an excellent role model of a scientist,” he says. “I have learned so much from him.”
Knopf left Switzerland to become a postdoctoral research assistant under the direction of atmospheric chemist Allan Bertram at the University of British Columbia in Canada (2003―2006).
“Allan Bertram made be a better chemist, appreciating kinetics, and guided me towards the professional life as a scientist,” says Knopf.
Bertram’s research group now shares research interests with Knopf’s group at Stony Brook: the physics and chemistry of the atmosphere, aerosol particles, heterogeneous atmospheric chemistry, mass spectrometry, and ice nucleation.
Looking back over his student years, says Knopf, “nowadays I realize that I was blessed having wonderful advisors and mentors along my way that ultimately led to a scientific career.”
Oceans Beckon
During his last postdoc year, he started to look at the ocean as a source of INPs―a sort of vast snow-making machine, driven in the same way that bacteria mixed with sprayed water are at the heart of making snow on ski slopes.
It was the ocean-INP thread of inquiry that led Knopf in 2006 to Stony Brook University and its aptly, inclusively named School of Marine and Atmospheric Sciences.
“If I go there,” he remembers thinking to himself, “I could understand the ocean better and the ocean-atmosphere interaction.”
Knopf says now, “I realized I would have all these experts. There was everything there.”
A Knack for Tinkering
“I had the luck of coming from an academic family,” Knopf says of his childhood in Schwetzingen, a small town just south of Heidelberg and Mannheim, the area’s big city. “I was always disposed to go after the science of things. (His father was a virologist and his mother a medical technician.)
“I had the luck of coming from an academic family. I was always disposed to go after the science of things.” – Daniel Knopf
As a boy, he built and flew remote-controlled planes, including one with a wingspan of 5 feet. He did the same with boats, becoming an expert in miniature combustion engines along the way.
“I was always building something,” says Knopf, whose handiness and speedy tinkering has served him well in the field.
It was during his master’s thesis on polar stratospheric clouds that Knopf realized his knack for tinkering also paid scientific dividends.
“I was really quick at translating a scientific question into an experiment,” he says.
‘A Lot of Gear’
That tinkerer’s impulse served Knopf well, for instance, during the 2019 aerosol-ice nucleation experiments at SGP.
“There was a lot of gear,” both offline and online, he says, since the campaign focused on recording the number, chemical composition, mixing state, and size of aerosol particles―key factors that impact ice nucleation.
There was a lot of gear also because of a key difference between Knopf’s SGP small ARM campaign and others like it: basing the study on the total captured ambient aerosol, and not just a fraction of it. The resulting aerosol particle data are now being used to test parameters designed to predict ice nucleation numbers in models.
Online instruments, fed by two high-volume sampling stacks, included a pair of machines for measuring the composition of aerosol particles in a range of sizes.
Next time, Knopf plans to add an inlet stack that captures and measures uncommonly large particles, in the range of 1 to 10 micrometers in diameter. It is these, he suspects, that can dominate ice nucleation.
Another online device measured biological particles―those, for example, from fungi, bacteria, and other sources. Two other instruments counted INPs in air samples that form ice at a given temperature and humidity.

In the SGP’s Guest Instruments Facility, Knopf and others kept an eye on aerosol particle sampling for four offline ice-formation experiments and for detailed offline single-particle chemical analysis.
Even today, Knopf puts some of the SGP aerosol samples through more precise analytical machines. One is the Scanning Transmission X-ray Microscope at the Advanced Light Source at Lawrence Berkeley National Laboratory in California. (He was there most recently in mid-October 2021.) Another is among the microscopy resources at the Environmental Molecular Sciences Laboratory, a DOE user facility in southeastern Washington state.
“You can really see the particles,” says Knopf of the aerosol-imaging acuity of such devices. “It’s what I always dreamed of.”
Getting Outside
In the end, it was a dream of snow and clouds and from whence they came that turned Knopf from particle physics to the physics and chemistry of the particles that make ice.
Oh: and the particles that make snow.
“Science is time consuming, but I still like skiing,” says Knopf of his present outdoors life, biking and hiking included. “I like getting out with the family.”
His wife is an animation director and visual communication designer.
Her artistic sensibilities “give me quite a balance,” says Knopf, whose life outside work that encompasses trips to art galleries in New York City, music of all kinds, wide-ranging reading, and friendly policy debates.
Then there is the middle-school daughter who excels at math, but who lately is drawn to acting over science.
“She thinks acting is cooler,” says Knopf. “We’ll see who wins.”
# # #Author: Corydon Ireland, Science Writer, Pacific Northwest National Laboratory
This work was supported by the U.S. Department of Energy’s Office of Science, through the Biological and Environmental Research program as part of the Atmospheric System Research program.

