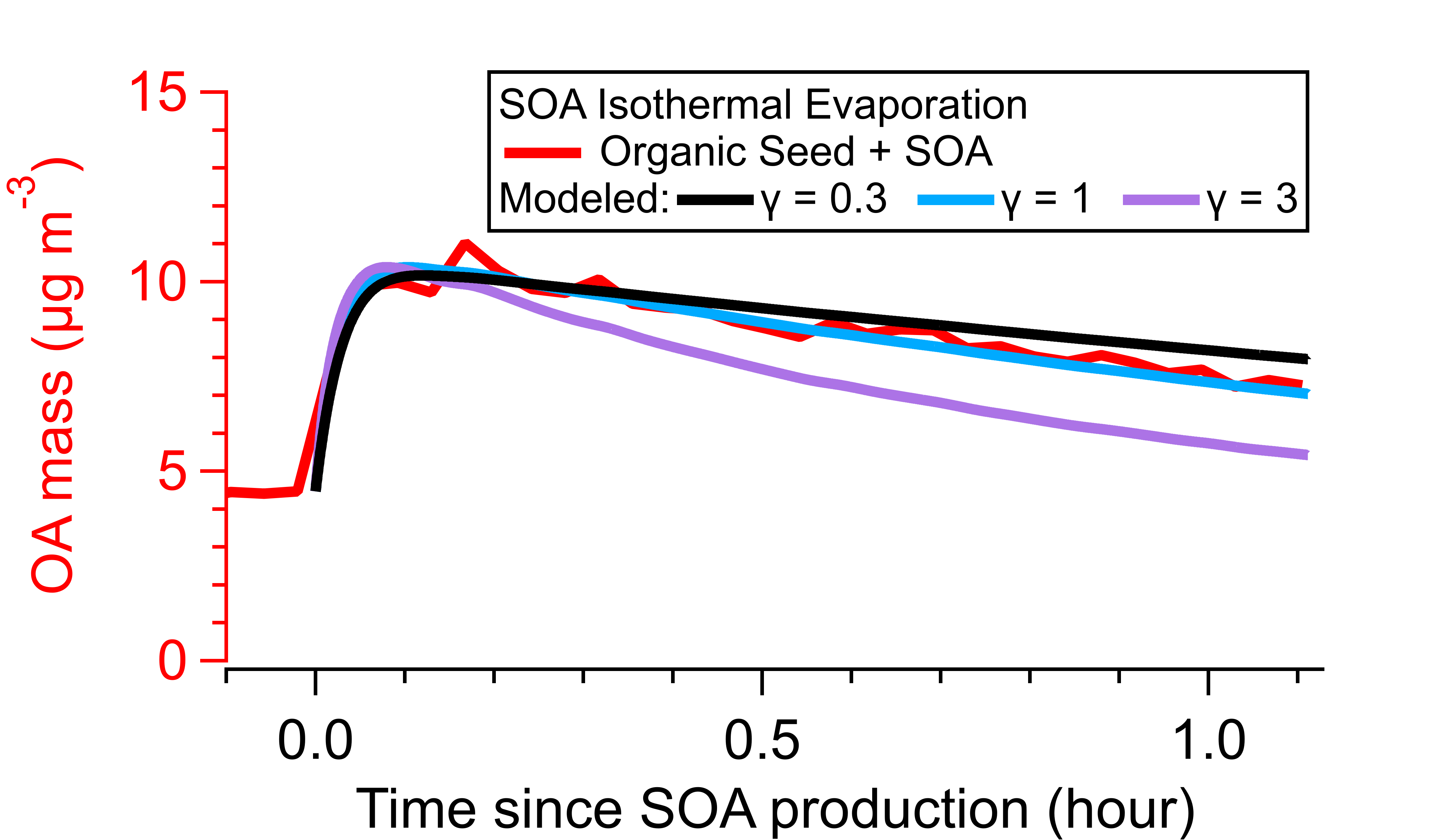
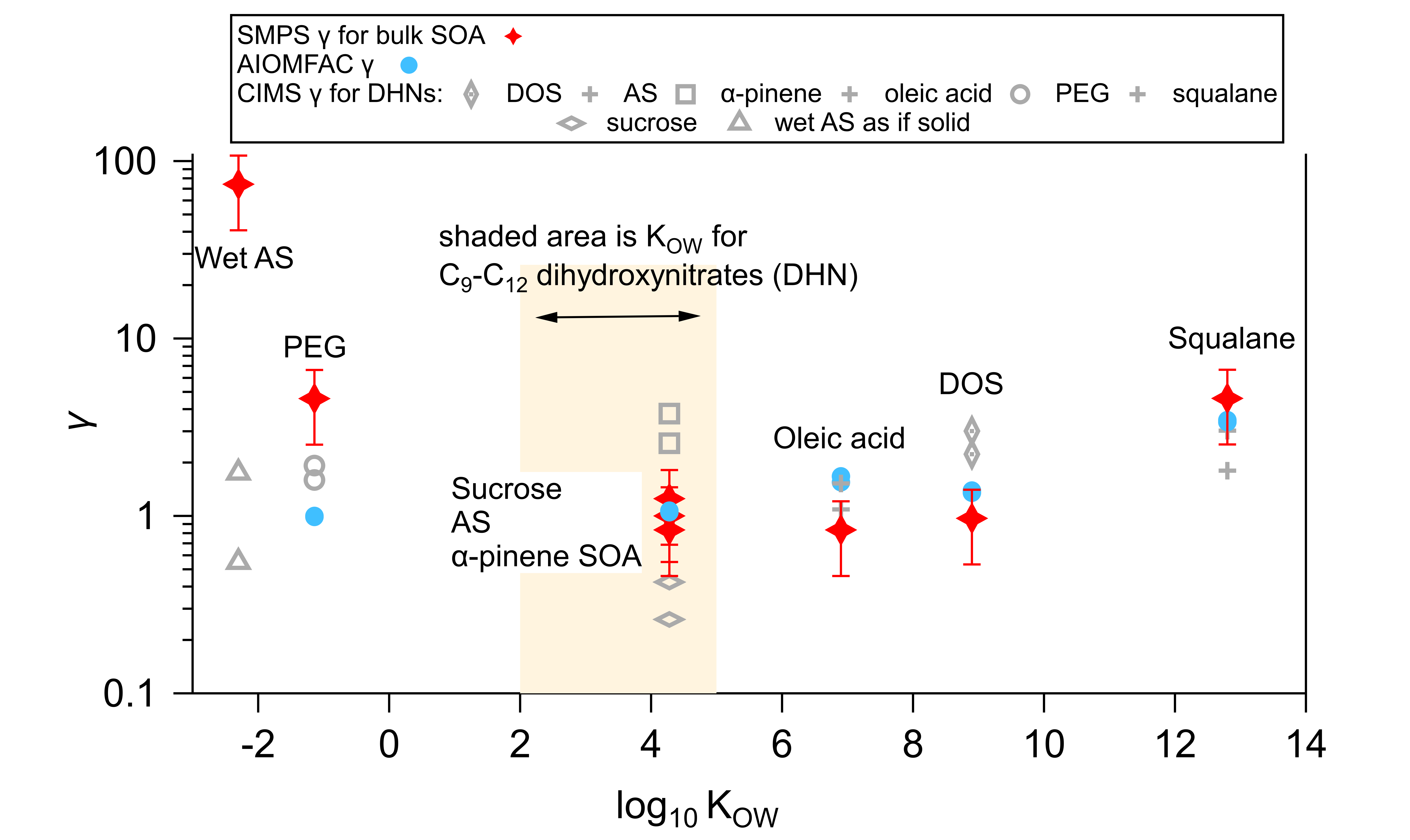
Determining activity coefficients of SOA from isothermal evaporation in a laboratory chamber
Submitter
Jimenez, Jose-Luis — University of Colorado
Ziemann, Paul J — University of Colorado
Area of research
Aerosol Properties
Journal Reference
Science
Activity coefficients (γ), a measure of non-ideal molecular interactions, were quantified using gas- and particle-phase observations of chamber SOA formation and isothermal evaporation for a wide range of seed particles. Increasing γ were observed as the polarity of the generated SOA molecules and pre-existing seed diverged.
Impact
Non-ideal molecular interactions in aerosol particles influence the partitioning of semi-volatile organic compounds (SVOCs). However, few direct measurements exist that determine activity coefficients (γ), which quantify nonideality, for individual oxidized organic compounds in different atmospheric mixtures. Our results demonstrate a method to quantify non-ideal behavior, and show that it can occur in multicomponent mixtures and therefore influence OA formation, evolution, and lifetimes. The inclusion of γ in estimating G/P partitioning is crucial for accurately predicting aerosol mass concentration and composition.
Summary
Effective saturation concentrations (c*) and activity coefficients (γ) of SVOCs in different seed particles were inferred using a combination of novel laboratory methods using environmental Teflon chambers and a model/fitting framework. Measurements were conducted under dry and humid conditions using seeds of different properties that are atmospherically relevant. The study uses a new “fast burst” method for quickly producing atmospherically relevant oxidized compounds that allows for a decoupling of the timescales of reaction (10 s), condensation (100s of s), vapor wall loss (1000 s), and net particle evaporation (several 1000s of s), together with state-of-the-art gas (NO3- CIMS) techniques capable of making fast (seconds) molecular-level measurements and standard detection particle methods (scanning mobility particle sizer) for tracking slower particle evaporation. Investigations of the isothermal evaporation of the bulk SOA, treating particles as a single liquid phase, showed that c* and γ were smaller when the SVOC and the seed particle had similar polarity (similar lipophilicity or hydrophilicity), otherwise c* and γ increased when the vapor and the particle were dissimilar, reflecting less favorable interactions. γ tends to increase from ~1 to ~5 as the organic seeds and the SOA have more differing polarities. High computed γ =74 for a wet ammonium sulfate/SOA system indicates phase separation. Peak SOA formation generally decreases as γ increases, which is consistent with more favorable partitioning of SVOC compounds when the seed or absorbing phase has more similar polarity to the partitioning compounds. γ for some individual species was also quantified based on gas-phase CIMS measurements of c*. However, the bulk SOA γ cannot be explained by the simplified speciated SVOC-seed interactions, suggesting incomplete compositional understanding of the bulk SOA.