Chemical transport models often underestimate aerosol acidity in remote regions of the atmosphere
Submitter
Jimenez, Jose-Luis — University of Colorado
Campuzano-Jost, Pedro — University of Colorado-Boulder
Area of research
Aerosol Processes
Journal Reference
Science
Measurements of aerosol pH and ammonium neutralization from several aircraft campaigns around the globe are presented and compared to outputs of several different chemical transport models. Measurements show increasing acidity with remoteness, at all altitudes, with pH decreasing from about 3 to about −1. Overall, the chemical transport models typically predict less acidic aerosol in the most remote regions.
Impact
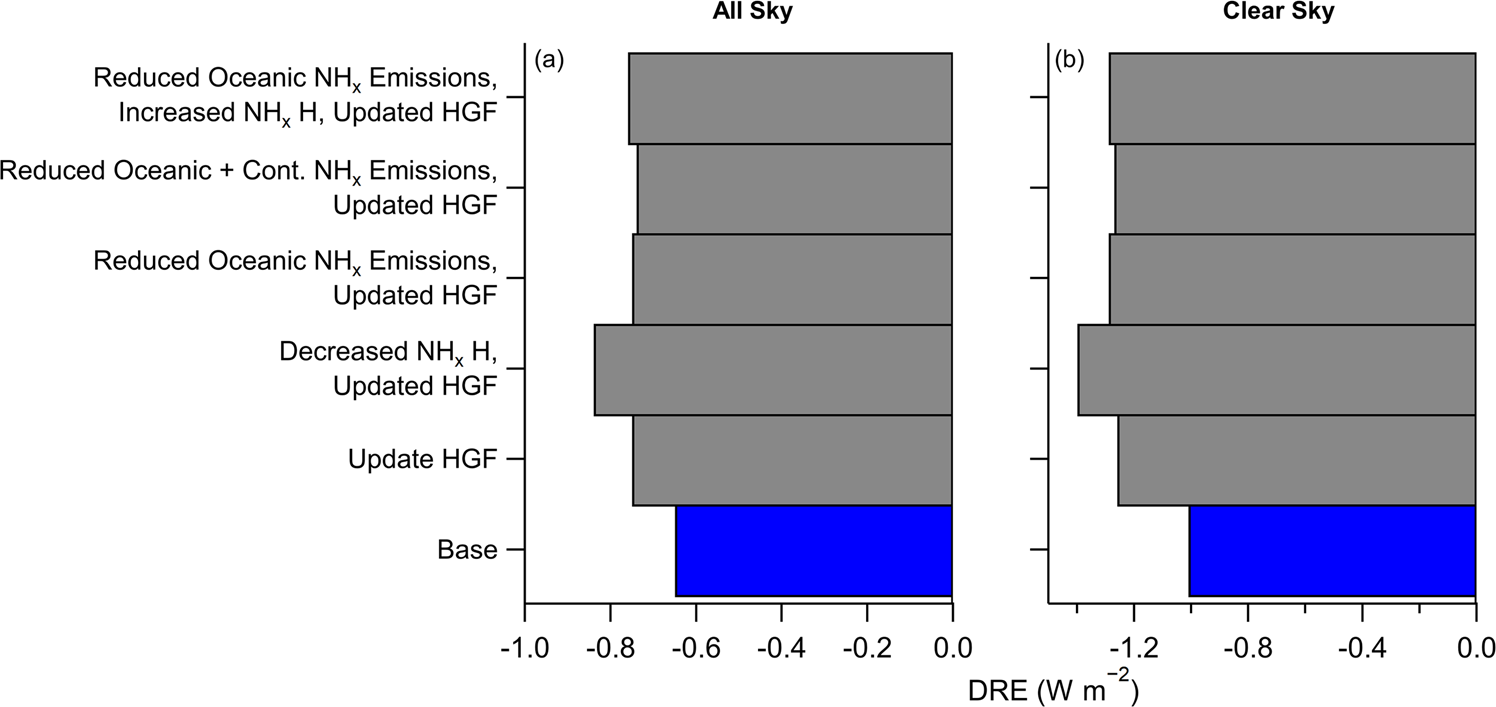
The inorganic fraction of fine particles affects numerous physicochemical processes as well as the radiative balance in the atmosphere. However, there is large uncertainty in its burden and composition due to limited global measurements. This survey shows that there are often large mismatches between measurements of aerosol acidity and predictions from many chemical transport models for remote regions. These discrepancies are likely to have substantial effects on model estimates of direct radiative cooling as well as chemical processes related to particles.
Summary
There is large uncertainty in the chemical composition of inorganic PM1 (submicron aerosols), due to uncertainty in emissions and lifetime of the precursor gases (specifically ammonia) and lack of measurements covering large swaths of the troposphere. Therefore, in this study we present observations from 11 different aircraft campaigns from polluted to the most pristine locations and investigate how aerosol pH and ammonium balance change from polluted to remote regions, such as over the oceans. Ammonium balance (NH4_Bal) is the fractional charge neutralization of nitrate, sulfate, and chloride by ammonium. We found a strong correlation of pH and NH4_Bal with inorganic PM1 mass concentration, indicating that as the air parcels are transported away from strong ammonia source regions (BB, agriculture, and anthropogenic activities), the continued production of sulfuric acid dominates the inorganic aerosol composition, leading to lower NH4_Bal and more acidic aerosol. We compare these observations against nine widely used chemical transport models (CTMs) and find that the simulations show more scatter (generally R2 < 0.50) and typically predict less acidic aerosol in the most remote regions. The likely reasons for the differences include: (1) too high ammonia emissions in the CTMs, especially over oceanic environments, (2) inefficient removal of ammonia leading to modeled lifetimes that are too long, and/or (3) assumption of internal mixing state of inorganic aerosol with sea-salt. In addition to potentially misrepresenting aerosol chemistry, these differences in observations and predictions are likely to result in underestimating the model-predicted direct radiative cooling effect for sulfate, nitrate, and ammonium aerosol by 15–39% (due in large part to underestimation of particle hygroscopicity).