Chemical Diffusivity and Viscosity of Secondary Organic Aerosols
Submitter
Zelenyuk-Imre, Alla — Pacific Northwest National Laboratory
Area of research
Aerosol Properties
Journal Reference
Science
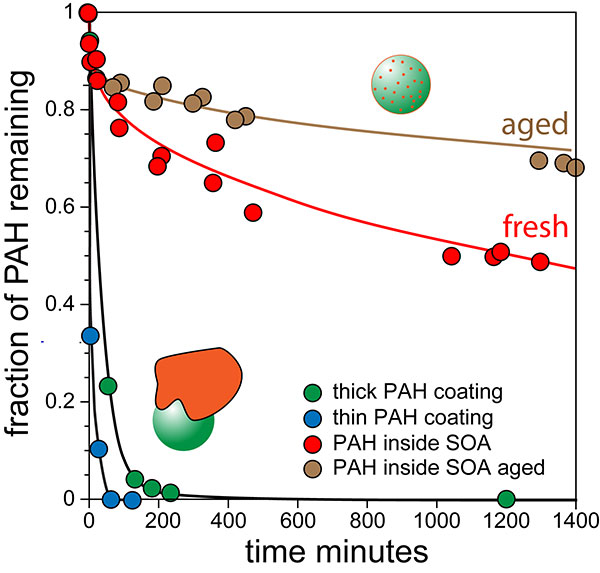
Secondary organic aerosols (SOA) comprise a majority of the atmospheric aerosol mass. SOA particles reflect solar radiation, serve as cloud condensation nuclei, are a chief vector for the long-range transport of pollutants, and thereby play an important role in climate and health. The properties, formation mechanisms, and temporal evolution of SOA have been the subject of intense laboratory and field research over the past 20 years. Despite this enormous effort, modeled SOA loadings have persistently failed to reproduce field observations. Due to lack of data, these models were constructed under the assumption that SOA particles are low-viscosity solutions. This paper presents the first direct measurements of the chemical diffusivity and viscosity of SOA particles thanks to a new approach developed by a team of scientists at Pacific Northwest National Laboratory (PNNL), University of Washington, and Imre Consulting. The team doped atmospherically important SOA particles with tracer molecules and measured their diffusion rate as they slowly worked their way out of these particles. Knowing the diffusion rate, the scientists calculated the particle's viscosity.
“Over the past two years, we have shown that long-standing assumptions about the most fundamental properties of SOA particles—phase and volatility—are wrong. Here, for the first time, we quantified chemical diffusivity in SOA particles and showed that SOA viscosity is larger—a million times higher than assumed,” said lead author Dr. Alla Zelenyuk, scientist at PNNL.
Impact
In this first-of-its-kind study, the team began by creating ~100-nanometer pyrene-doped SOA particles. The particles were generated via the reaction of ozone with alpha-pinene, the chemical that gives pine trees their smell, in the presence of pyrene vapors. These particles contain a trace amount of the hydrophobic pyrene molecules that slowly diffuse out through the highly viscous particles. The team used a single particle mass spectrometer, SPLAT II, at the U.S. Department of Energy’s Environmental Molecular Science Laboratory (EMSL), a DOE user facility located at PNNL, to follow the temporal evolution of particle size, shape, morphology, density, and composition. This multi-dimensional particle characterization approach allowed them to measure the rate with which the pyrene molecules diffuse through the particles and evaporate when they reach the surface, from which the team calculated the viscosity of the particles.
“We showed that the particles have viscosity similar to that of tars,” said Zelenyuk. “Moreover, with time, they harden, becoming more viscous by a factor of 3 in a day.”
In addition to answering key questions about the properties of the atmospherically important SOAs, the team demonstrated the effectiveness of their new methodology. “We showed that it is possible to create doped nanoparticles and measure very high viscosity on the time scale of hours to days, instead of years,” said Zelenyuk.
Summary
Until now, assumptions about the volatility and viscosity of SOAs in climate and process models have made it impossible to correctly model how the particles affect climate and human health. With the development of new approaches and precise characterization instruments, scientists have disproved those common assumptions. In this current study, the team provided atmospheric modelers data that are invaluable for correctly portraying the SOA particles and their effects in different scenarios.