An ASR project combines field measurements, lab experiments, and computer modeling

So it is important to ask: How do these particles form, and how do they grow?
The growth of newly formed particles is particularly important. On its way to CCN-size, a particle needs to grow fast enough in order to avoid being swept away by collisions with other particles in the air. It’s a constant race for survival to climate relevancy, and it’s not well understood; nor is it well represented in aerosol-climate models of the Earth’s climate systems.
Addressing these gaps in knowledge is a project funded since 2013 by the Atmospheric System Research (ASR) program at the U.S. Department of Energy (DOE). It’s the brainchild of two veterans of the NPF puzzle, atmospheric chemist James N. “Jim” Smith of the University of California, Irvine, and atmospheric modeler Jeffrey R. Pierce at Colorado State University.
In search of insights into NPF, they combine computer modeling with laboratory measurements and with field measurements funded by the DOE.

Smith’s group uses field data as well as data from Irvine’s atmospheric reaction chamber. Its focus is measuring atmospheric nanoparticles down to 10 nm in size. Pierce’s team develops computer models that represent aerosol growth dynamics. Ultimately, these models will be integrated into regional and global climate models.
Other collaborators fill out a big team that Smith said will “lift the haze” on how new particles are formed. David R. Hanson, a physical chemist at Augsburg College, provides expertise on a class of gases called amines, which are important to NPF. Mechanical engineer Peter H. McMurry, though now retired from the University of Minnesota, weighs in as an expert in aerosol instrumentation.
Dramatic Atmospheric Events
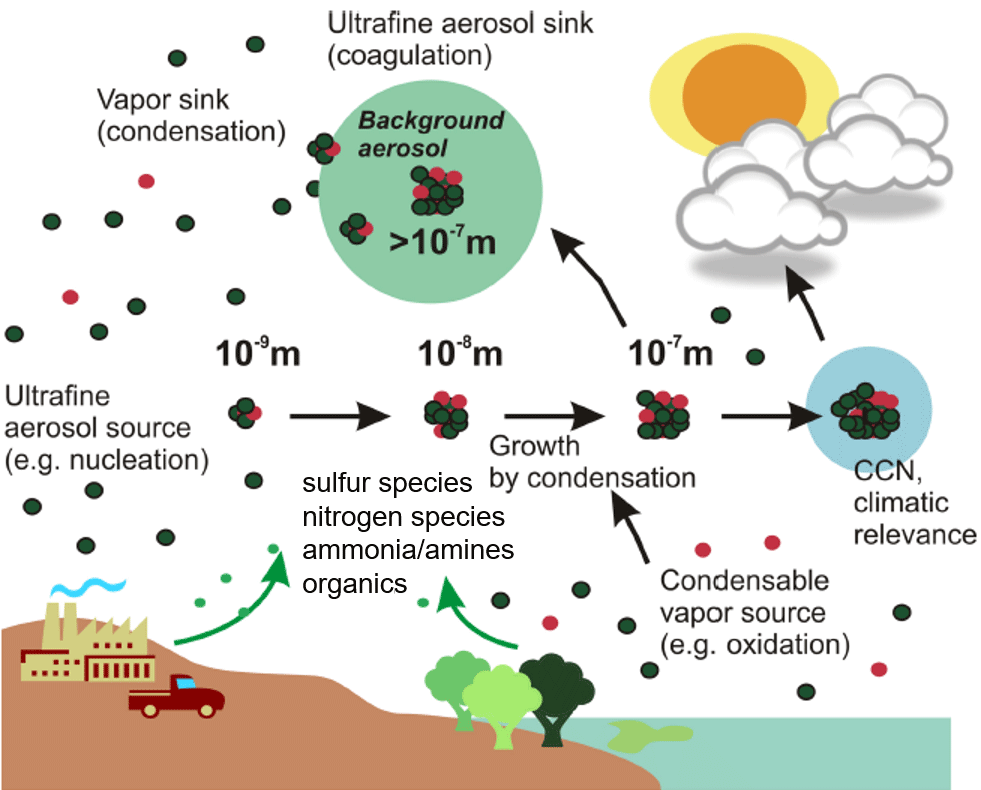
The formation of new particles in the atmosphere begins with a self-assembly process called nucleation. “Nucleating” vapors created from chemistry in the atmosphere may form nanometer-size particles. Their chemical precursors can be emitted from anthropogenic, or human-based, sources, like coal-fired power plants. But many of them come from natural sources like trees (largely as organic compounds) and oceans (in the form of sulfur-based compounds such dimethyl sulfide).
These natural vapors often combine with anthropogenic oxidants. NPF from this chemical soup is the dominant source of particles in the atmosphere, said Smith, in volumes “even greater than all the air-polluting factories and cars” in the world. And yet, he added, “we still don’t understand the process well enough to predict their occurrence.”
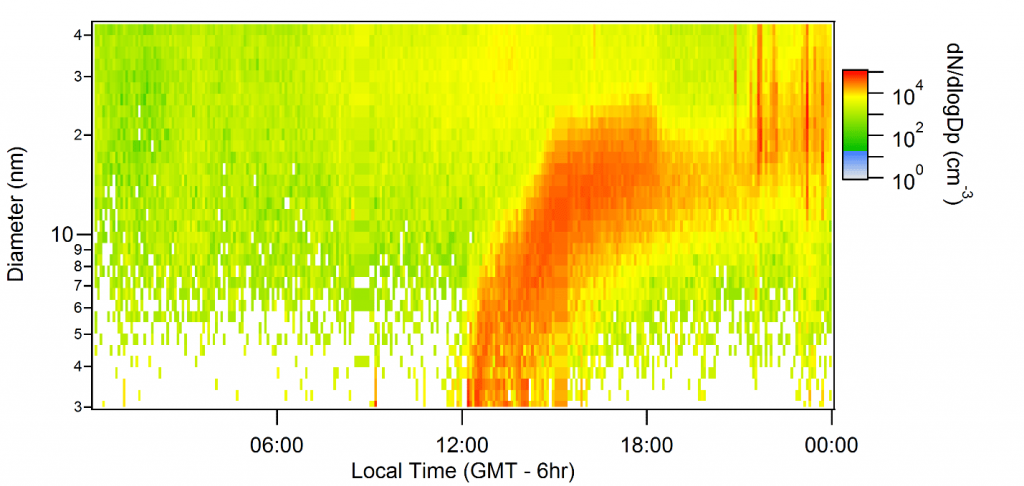
Plotting concentrations by particle size and time, scientists call these spontaneous emissions “banana events,” named after the curving, columnar appearance the new particles collectively take within minutes or hours. In one plot (pictured), the newly formed particles are 3 nm in diameter at noon, grow to 20 nm by 2 p.m., and by 6 p.m. are 30 nm in diameter.
Only a thousandth of these particles may become cloud condensation nuclei, the seeds upon which clouds are built. But that one in a thousand is important for its climatic effect, said Smith.
Lessons from the Oklahoma Skies
Little is known about how these particles form or grow, but a few things are certain. Chemicals that are “extra sticky,” like amines, play a role because of their ability to cling together to form low-volatility complexes. It is also certain that particle formation happens “pretty much everywhere” throughout the atmosphere, said Smith, though with very different frequency and rates, depending on location.
In the spring of 2013, during the New Particle Formation Study, he and others proved that by rigging condensation particle counters to a tethered balloon at the Southern Great Plains (SGP) site, a DOE Atmospheric Radiation Measurement (ARM) Climate Research Facility atmospheric observatory in Oklahoma.
The resulting data showed that once nanoparticles are formed, they are well mixed throughout the atmosphere’s boundary layer within an hour of formation. And not incidentally, said Smith, the instrument platform—a vivid red balloon that rose up to a kilometer in the sky—illustrates another instance of “DOE becoming extremely interested in unmanned platforms.”
The ultimate goal of combining NPF field and lab experiments with computer modeling is to predict how the future atmosphere will respond to such things as climate variability, land-use change, and improvements in (or degradation of) emissions controls in urban areas. That means modelers like Pierce have to figure out ways to represent NPF events in global earth system models.
One obstacle to modelers is representing the number of new particles within a given cubic centimeter of atmosphere—not just the total mass of particles, said Smith, who co-leads ASR’s aerosol processes working group. In turn, modelers have to take the properties of the tiny particles the Smith group studies and move them into “a global spatial scale,” he said.
An ASR Paper Trail
The Smith-Pierce proposal, which is funded for one more year, uses a mechanistic model for nanoparticle growth called MABNAG, or the Model for Acid-Base Chemistry in Nanoparticle Growth.
Two papers—one published last year and the other slated for publication this summer—illustrate the direction Smith and Pierce have taken the project in the last few years.
The first paper, in Atmospheric Chemistry and Physics, shows how the MABNAG model does a good job of something called “chemical closure.” That makes it possible to predict the measured evolution of particle growth and competition by using current theory and measured gas-phase concentration.
“This means we are making progress in modeling NPF,” said Smith.
The paper, led by first author Anna L. Hodshire, a Pierce protégé and doctoral student at Colorado State, models both the composition and the growth rate of the particles formed at the SGP site. The model predicted how gases are taken up in the particles, how the particles evolve, and how big they become.
In just one hour, as the banana-event plot illustrates, a particle can grow 2 to 10 nanometers. That makes measuring the composition of these growing particles a challenge in the field because enough mass has to be collected “at the young and low end,” said Smith. “We’re the only group in the world that collects and measures the mass and composition of these very young particles.”
The second paper, with Irvine postdoc Haihan Chen as the lead author, was presented in PowerPoint form earlier this spring at the ARM/ASR Joint User Facility and Principal Investigator Meeting in Tysons, Virginia. It not only established that NPF occurs throughout the atmosphere. It also indicated that newly formed particles “soak up water like a sponge,” along with certain gases.
That is “even more interesting,” said Smith, the paper’s corresponding author, and means that it is better to think of a nanoparticle in ambient air as a water droplet rather than as a speck of dust.
On the modeling side, Smith credited Pierce and Hodshire with some “pretty cool modeling that showed some of the actual implications of the wetness of nanoparticles.”
Last year, Smith joined a few other investigators for a follow-up study of particle size distribution and chemical composition at SGP once again, this time as participants in the Holistic Interactions of Shallow Clouds, Aerosols, and Land-Ecosystems (HI-SCALE) field campaign.
Currently, Pierce’s group is using Hodshire’s work to expand his grasp of the mechanisms of particle formation. He also continues to connect Smith’s lab and field observations to global and regional climate models.
As for Smith’s group—it is in the laboratory, investigating another corner of the NPF puzzle: how low temperature and humidity play a role in new particle growth.
Fort Collins and Irvine may be geographically far apart, but distance is no barrier to this ASR collaboration. “Even though Jeff and I are working in different states,” said Smith, “we are really working closely together.”
References:
Hodshire AL, MJ Lawler, J Zhao, J Ortega, C Jen, T Yli-Juuti, JF Brewer, JK Kodros, KC Barsanti, DR Hanson, PH McMurry, JN Smith, and JR Pierce. 2016. “Multiple new-particle growth pathways observed at the US DOE Southern Great Plains field site.” Atmospheric Chemistry and Physics, 16(14), 10.5194/acp-16-9321-2016.
Riipinen I, JR Pierce, T Yli-Juuti, T Nieminen, S Hakkinen, M Ehn, H Junninen, K Lehtipalo, T Petaja, J Slowik, R Chang, NC Shantz, J Abbatt, WR Leaitch, V-M. Kerminen, DR Worsnop, SN Pandis, NM Donahue, and M Kulmala. 2011. “Organic condensation: a vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations.” Atmospheric Chemistry and Physics, 11(8), 10.5194/acp-11-3865-2011.
# # #
This work was supported by the U.S. Department of Energy’s Office of Science, Office of Biological and Environmental Research as part of the Atmospheric System Research Program.

